Calculate the Energy of the Photon Emitted for Transition a
You can use the Rydberg formula to calculate the change in energy. Rydbergs original formula was in terms of wavelengths but we can rewrite it to have the units of energy.
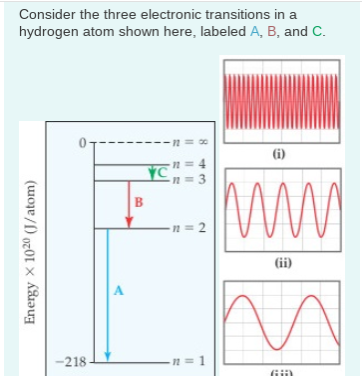
Solved Part A Calculate The Energy Of The Photon Emitted Chegg Com
The formula then becomes.

. Two-photon absorption TPA or 2PA or two-photon excitation or non-linear absorption is the simultaneous absorption of two photons of identical or different frequencies in order to excite a molecule from one state usually the ground state to a higher energy most commonly an excited electronic stateAbsorption of two photons with different frequencies is called non-degenerate.

Calculate The Wavelength Of A Photon Emitted When An Electron In H Atom Maker A Transition Youtube

Bohr Calculation Example 1 Find De And The Wavelength Of The Photon Emitted Youtube
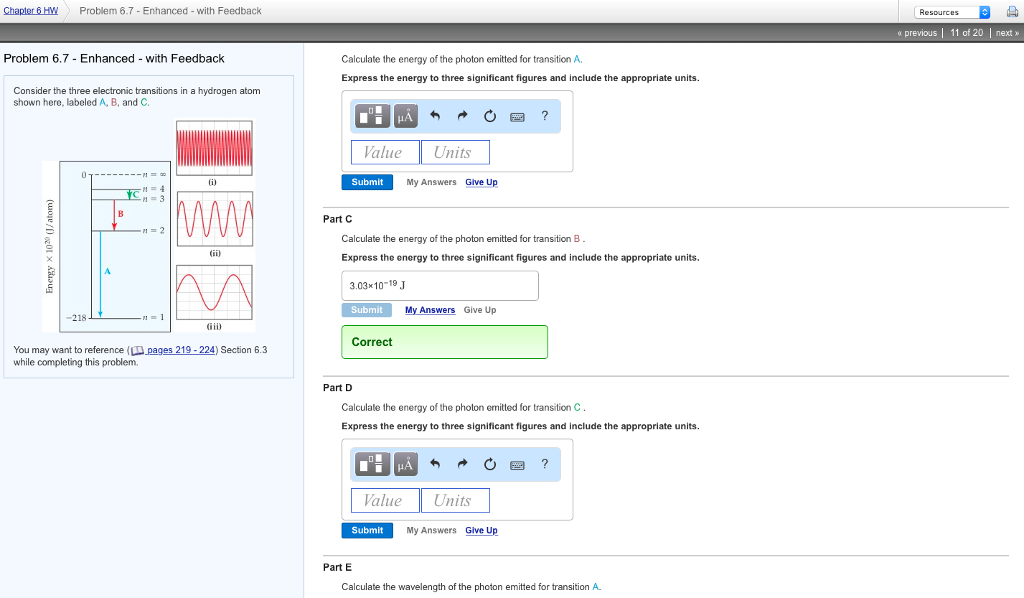
Solved Chapter 6hw Problem 6 7 Enhanced With Feedback A Chegg Com

What Is The Wavelength Of A Photon Emitted During A Transition From N 5 State To The N 2 Youtube
No comments for "Calculate the Energy of the Photon Emitted for Transition a"
Post a Comment